Out Of This World Info About Why Are Electrons Lazy

1.3 Atomic Theory. Ppt Download
Electrons
1. Unpacking Electron "Laziness"
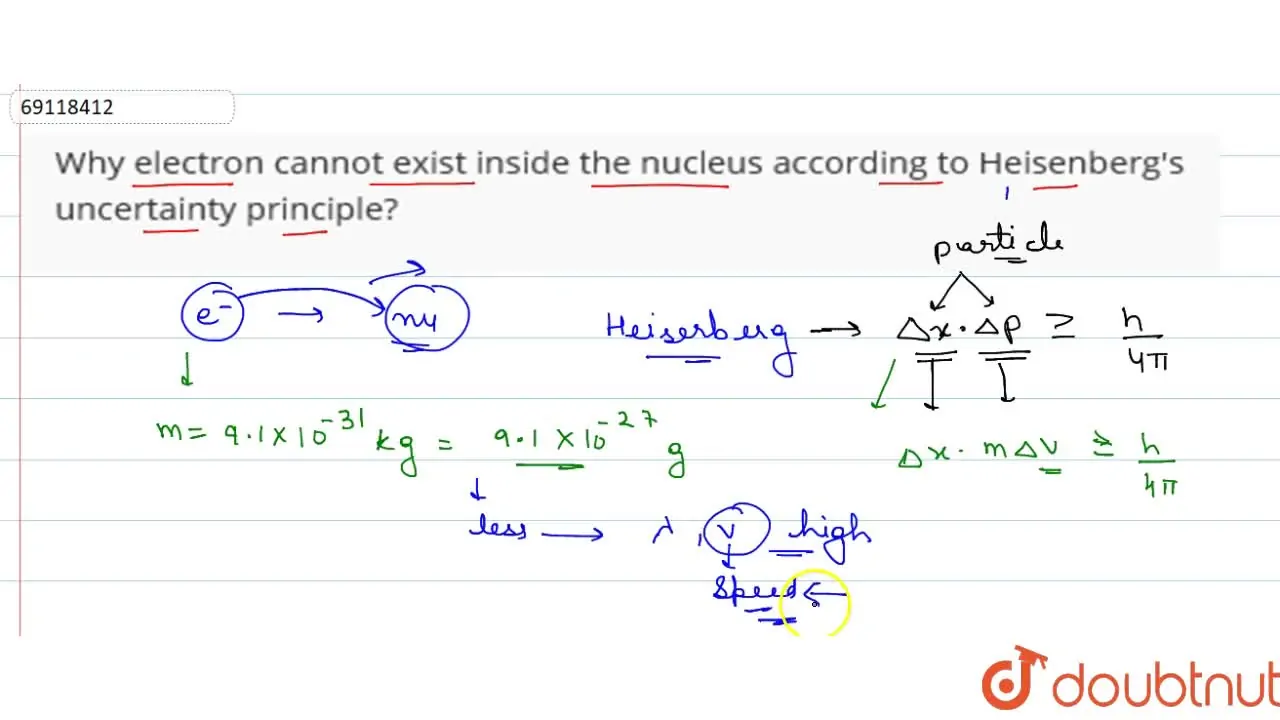
We often hear about electrons being "lazy," but is that really accurate? I mean, they're not exactly lounging around with tiny electron-sized cups of coffee, are they? The truth is, calling them lazy is a bit of a simplification, a way of making a complex idea easier to grasp. It's more about their inherent desire to be in the lowest energy state possible. Think of it like this: if you had the choice between climbing a mountain or relaxing on a comfy couch, which would you pick? Most of us would choose the couch, right? It requires less effort.
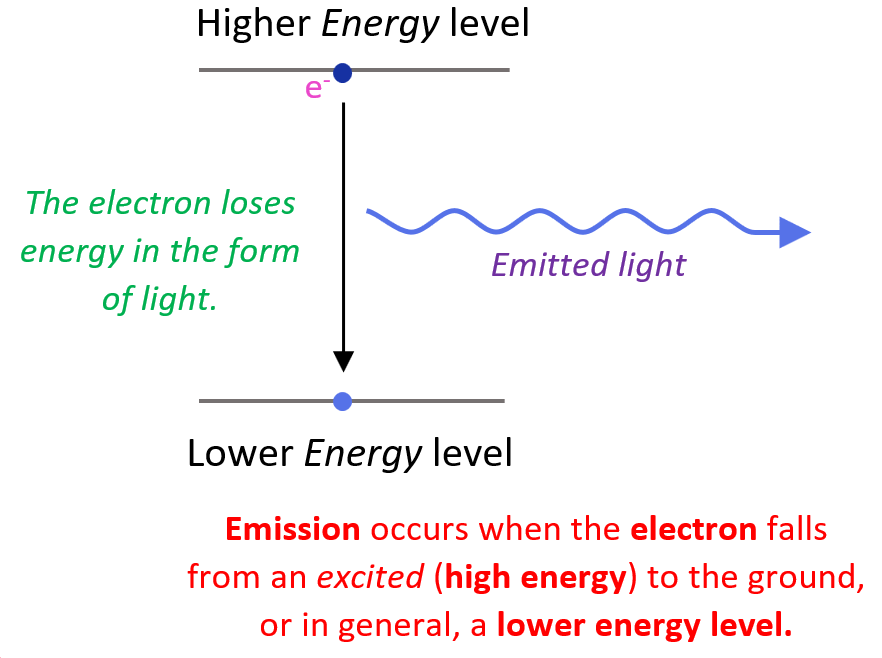
Electrons are similar. They "prefer" to occupy the lowest energy levels within an atom. These are the energy levels closest to the nucleus. Moving to a higher energy level requires an input of energy, kind of like needing that motivation to actually start climbing that mountain. An electron will only jump to a higher level if it absolutely has to — if it absorbs a photon of light with the precise amount of energy needed, for example.
But what happens when that external energy source disappears? You guessed it! The electron, like a tired climber reaching the summit, wants to get back down to a lower energy state. It will release the excess energy, often in the form of another photon of light, and return to its original, more stable position. This is the fundamental principle behind how atoms emit light — the very thing that allows us to see the world around us. Pretty cool, huh?
So, the next time you hear someone say electrons are lazy, remember it's just shorthand. They are simply obeying the laws of physics, striving for the most stable and energy-efficient arrangement possible. It's not laziness; it's just good science!
Delving Deeper
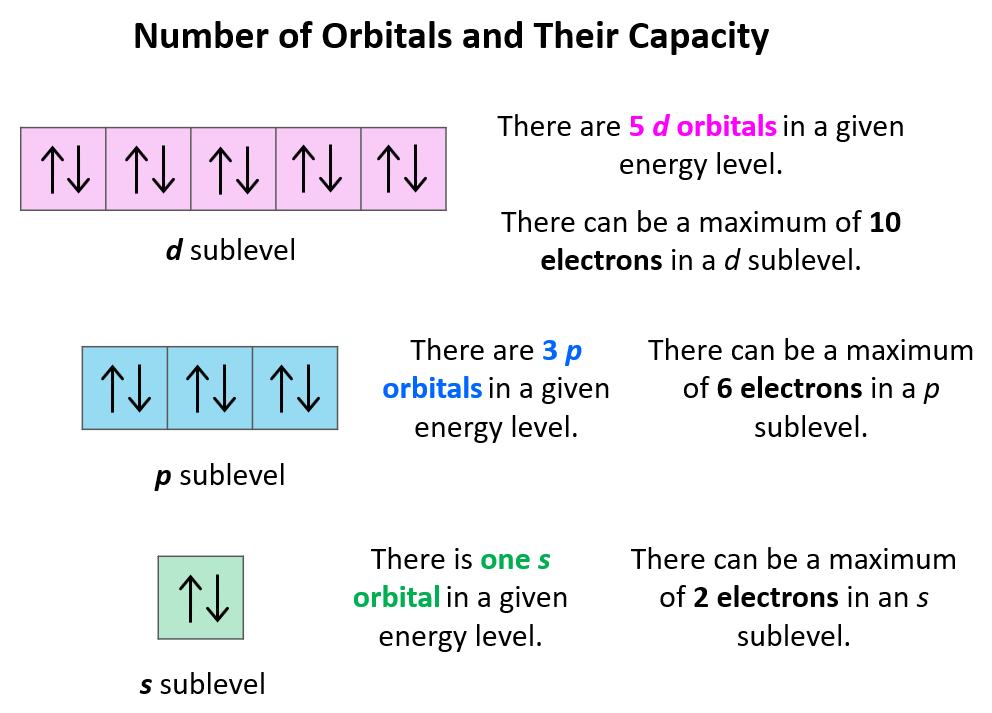
2. Understanding the Physics Behind Electron Preference
Okay, so we've established that electrons like to hang out in the lowest energy state, but why? What's so appealing about this low-energy lifestyle? The answer lies in the fundamental forces governing the universe. Specifically, the electromagnetic force. The positively charged nucleus of an atom attracts the negatively charged electrons. This attraction is strongest when the electrons are closest to the nucleus, hence the preference for lower energy levels. It's like gravity, but with charges! The closer you are, the stronger the pull.
Imagine an electron far away from the nucleus. It's still attracted, but the attraction is weaker. It's like trying to hear someone whisper across a crowded room. Now imagine that electron much closer to the nucleus. The attraction is much stronger, like having that person whisper directly into your ear. This stronger attraction represents a lower energy state because the electron is more tightly bound to the atom. It would require more energy to pull it away.
Think of it like a ball rolling down a hill. The ball naturally rolls to the lowest point, where it has the least potential energy. Similarly, electrons "roll" towards the lowest energy levels around the nucleus. This tendency to minimize energy is a universal principle, applying to everything from atoms to galaxies. It's a key driver of how the universe organizes itself.
Therefore, its not a matter of electrons being unmotivated to do anything. Its that their position around the nucleus is dictated by a series of physical laws, which ultimately determine an electrons location and energy. They always follow the path of least resistance, not out of laziness, but out of necessity!
Electron "Laziness" and Chemical Bonding
3. How Electron Energy Levels Influence Molecular Interactions
This "laziness," this drive to minimize energy, is actually the engine that drives chemical bonding. When atoms come together to form molecules, they do so in a way that lowers the overall energy of the system. Electrons are rearranged, shared, or transferred between atoms to achieve this more stable configuration.
Consider a simple example: the formation of a water molecule (H2O). Two hydrogen atoms each share an electron with an oxygen atom, creating covalent bonds. This sharing of electrons allows all three atoms to achieve a more stable electron configuration, resembling the electron configuration of a noble gas. In other words, by bonding together, the atoms can effectively "fill" their outer electron shells, which is a state of lower energy and greater stability.
The type of bond formed (ionic, covalent, metallic, etc.) depends on the electronegativity of the atoms involved. Electronegativity is a measure of how strongly an atom attracts electrons. Atoms with high electronegativity "want" electrons more than atoms with low electronegativity. This difference in electronegativity dictates how electrons are distributed in a chemical bond.
So, electron "laziness" at the atomic level leads to the formation of chemical bonds at the molecular level. These bonds, in turn, determine the properties of matter — its melting point, boiling point, reactivity, and everything else. Everything we see and touch is a consequence of electrons trying to find their most comfortable, low-energy arrangement. It's amazing, really!

Zinc Electrons Level At Armanda Rael Blog
Beyond the Atom
4. Electron Behavior in Solids
Let's zoom out a bit and think about what happens when you have a whole bunch of atoms packed together in a solid. In a solid, the individual atoms interact with each other, and their electron energy levels become broadened into energy bands. Instead of having discrete energy levels, electrons can now occupy a range of energies within these bands.
This band structure is crucial for understanding the electrical properties of materials. In metals, the highest energy band is only partially filled, meaning that electrons can easily move from one energy level to another within the band. This allows them to conduct electricity very efficiently. Its like having a highway with plenty of open lanes; traffic flows smoothly.
In insulators, on the other hand, the highest filled band (the valence band) is completely filled, and there is a large energy gap (the band gap) between the valence band and the next available band (the conduction band). This means that electrons need a significant amount of energy to jump across the band gap and become conductive. It's like having a huge roadblock on the highway; traffic grinds to a halt.
Semiconductors are somewhere in between metals and insulators. They have a smaller band gap than insulators, so electrons can jump across the gap with a moderate amount of energy. This allows their conductivity to be controlled by factors like temperature, light, or impurities. This controllable conductivity is what makes semiconductors so useful in electronic devices.
Bohr Model Of The Hydrogen Atom Chemistry Steps
"Lazy" Electrons and Technology
5. From Transistors to Lasers
All of our modern technology, from smartphones to computers to lasers, relies on our understanding and ability to manipulate electron behavior. Transistors, the building blocks of modern electronics, work by controlling the flow of electrons through a semiconductor material. By applying a voltage to a transistor, we can switch it on or off, allowing us to perform logical operations.
Lasers, on the other hand, use the principle of stimulated emission to generate a highly focused beam of light. This process involves exciting electrons to higher energy levels and then triggering them to release photons of light with the same wavelength and phase. The resulting light is coherent and can be used for a wide range of applications, from barcode scanners to surgical tools.
Even solar cells harness the "laziness" of electrons. They absorb sunlight, which excites electrons in a semiconductor material. These excited electrons then flow through an external circuit, generating electricity. It's a way of converting sunlight into electrical energy, all thanks to the inherent properties of electrons.
So, while we might jokingly call electrons lazy, their behavior is actually the foundation of much of the technology we rely on every day. By understanding their desire to minimize energy, we have been able to create amazing devices that have transformed our world. And that's definitely not lazy!
